Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O -75MoO
-75MoO binary glasses composed of isolated MoO
binary glasses composed of isolated MoO

Masuno, Atsunobu*; Munakata, Sae*; Okamoto, Yoshihiro; Yaji, Toyonari*; Kosugi, Yoshihisa*; Shimakawa, Yuichi*
Inorganic Chemistry, 63(12), p.5701 - 5708, 2024/03
Times Cited Count:0 Percentile:0.05(Chemistry, Inorganic & Nuclear)Transparent and brown La O
O -MoO
-MoO binary glasses were prepared in bulk form using a levitation technique. The glass-forming range was limited, with the primary composition being approximately 25 mol% La
binary glasses were prepared in bulk form using a levitation technique. The glass-forming range was limited, with the primary composition being approximately 25 mol% La O
O . This glass exhibited a clear crystallization at 546
. This glass exhibited a clear crystallization at 546  C, while determining its glass transition temperature was difficult. Notably, despite its amorphous nature, the glass possessed a density and packing density comparable to those of crystalline La
C, while determining its glass transition temperature was difficult. Notably, despite its amorphous nature, the glass possessed a density and packing density comparable to those of crystalline La Mo
Mo O
O . X-ray absorption fine structure and Raman scattering analyses revealed that the glass structure closely resembles La
. X-ray absorption fine structure and Raman scattering analyses revealed that the glass structure closely resembles La Mo
Mo O
O due to the presence of isolated MoO
due to the presence of isolated MoO
 units, whereas disordered atomic arrangement around La atoms was confirmed. The glass demonstrated transparency ranging from 378 to 5500 nm, and the refractive index at 1.0
units, whereas disordered atomic arrangement around La atoms was confirmed. The glass demonstrated transparency ranging from 378 to 5500 nm, and the refractive index at 1.0  m was estimated to be 2.0. The optical bandgap energy was 3.46 eV, which was slightly smaller than that of La
m was estimated to be 2.0. The optical bandgap energy was 3.46 eV, which was slightly smaller than that of La Mo
Mo O
O . Additionally, the glass displayed a transparent region ranging from 6.5 to 8.0
. Additionally, the glass displayed a transparent region ranging from 6.5 to 8.0  m. This occurrence results from the decreased diversity of MoO
m. This occurrence results from the decreased diversity of MoO units and connectivity of Mo-O-Mo, which resulted in the reduced overlap of multiphonon absorption. This glass formation, with its departure from conventional glass-forming rules, resulted in distinctive glasses with crystal-like atomic arrangements.
units and connectivity of Mo-O-Mo, which resulted in the reduced overlap of multiphonon absorption. This glass formation, with its departure from conventional glass-forming rules, resulted in distinctive glasses with crystal-like atomic arrangements.
Tonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 589, p.154862_1 - 154862_10, 2024/02
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)The dissolution behavior of FeUO compounds formed by a high-temperature reaction of UO
compounds formed by a high-temperature reaction of UO with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U
with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U O
O and Fe
and Fe O
O as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO
as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO
compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U
dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U O
O ).
).
Nagai, Takayuki; Hasegawa, Takehiko*
JAEA-Research 2023-008, 41 Pages, 2023/12
To reduce the risks posed by stored the high-level radioactive liquid waste (HAW), Tokai Vitrification Facility (TVF) is working to produce the HAW into vitrified bodies. With the aim of steady vitrification of HAW in TVF, the vitrification technology section has manufactured a new 3rd melter with an improved bottom structure and is working to verify the performance of this melter. In this study, solidified glass samples were taken from simulated vitrified bodies produced by flowing molten glass during the bottom drain-out test in the operation confirmation of the TVF 3rd melter. And the properties of the surface layer and fracture surface of the vitrified bodies were evaluated by using Raman spectroscopy, synchrotron radiation XAFS measurement, and laser ablation inductively coupled plasma atomic emission spectroscopy (LA ICP-AES) analysis. As a result of measuring the surface layer and fracture surface of the solidified samples produced on an actual scale, a slight difference was confirmed between the properties of the surface layer and those of the fracture surface. Since the chemical composition of these simulated vitrified bodies does not contain platinum group elements, it is expected that the glass structure of solidified glass samples is different from that of the actual vitrified body. However, this sample measuring was a valuable opportunity to evaluate samples produced by using the direct energized joule heating method. The properties of cullet used the operation confirmation of the TVF 3rd melter and the cullet of another production lot were measured and analyzed in the same manner under the measuring conditions of solidified glass samples. As a result, it was confirmed that cullet with different producing histories have different glass structures even with the same chemical composition, and that differences in glass structures remain in the glass samples after melting these cullet.
 Ga K-edge XANES study of Ga-exchanged zeolites at high temperatures under different atmospheres including vacuum, CO, and pressurized H
Ga K-edge XANES study of Ga-exchanged zeolites at high temperatures under different atmospheres including vacuum, CO, and pressurized H
Huang, M.*; Kinjo, Tetsuya*; Yasumura, Shunsaku*; Toyao, Takashi*; Matsumura, Daiju; Saito, Hiroyuki*; Shimizu, Kenichi*; Namiki, Norikazu*; Maeno, Zen*
Catalysis Science & Technology, 13(23), p.6832 - 6838, 2023/12
Times Cited Count:0 Percentile:0(Chemistry, Physical)Okazaki, Hiroyuki*; Idesaki, Akira*; Koshikawa, Hiroshi*; Matsumura, Daiju; Ikeda, Takashi*; Yamamoto, Shunya*; Yamaki, Tetsuya*
Journal of Physical Chemistry C, 127(49), p.23628 - 23633, 2023/12
Times Cited Count:0 Percentile:0(Chemistry, Physical)Matsumura, Daiju; Kimura, Yusaku*; Tsuji, Takuya; Mizuki, Junichiro*
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(5), p.296 - 299, 2023/11
no abstracts in English
Ikeda, Yoichi*; Umemoto, Yoshihiko*; Matsumura, Daiju; Tsuji, Takuya; Hashimoto, Yuki*; Kitazawa, Takafumi*; Fujita, Masaki*
Materials Transactions, 64(9), p.2254 - 2260, 2023/09
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary)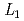 -edge XAFS measurement
-edge XAFS measurementTsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(4), p.214 - 217, 2023/08
no abstracts in English
Inagawa, Kohei*; Matsumura, Daiju; Taniguchi, Masashi*; Uegaki, Shinya*; Nakayama, Tomohito*; Urano, Junnosuke*; Aotani, Takuro*; Tanaka, Hirohisa*
Journal of Physical Chemistry C, 127(24), p.11542 - 11549, 2023/06
Times Cited Count:0 Percentile:0(Chemistry, Physical)Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:1 Percentile:44.81(Chemistry, Multidisciplinary)no abstracts in English
 -edge X-ray absorption fine structure study of
-edge X-ray absorption fine structure study of 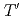 -type
-type 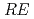
 CuO
CuO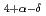 (
(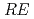 = Rare Earth); Toward unified understanding of electronic state of
= Rare Earth); Toward unified understanding of electronic state of 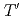 -type cuprate
-type cuprateChen, Y.*; Asano, Shun*; Wang, T.*; Xie, P.*; Kitayama, Shinnosuke*; Ishii, Kenji*; Matsumura, Daiju; Tsuji, Takuya; Taniguchi, Takanori*; Fujita, Masaki*
JPS Conference Proceedings (Internet), 38, p.011050_1 - 011050_6, 2023/05
 O
O ) by mechanochemical treatment
) by mechanochemical treatmentAkiyama, Daisuke*; Mishima, Tomoki*; Okamoto, Yoshihiro; Kirishima, Akira*
High Temperature Materials and Processes, 42(1), p.20220268_1 - 20220268_9, 2023/04
A powder mixture of UO and TiO
and TiO was mechanochemically treated in a planetary ball mill under Ar atmosphere for 1 h using a tungsten carbide vial and balls as the milling medium. Such mechanochemical (MC) treatment reduced the crystallinity of UO
was mechanochemically treated in a planetary ball mill under Ar atmosphere for 1 h using a tungsten carbide vial and balls as the milling medium. Such mechanochemical (MC) treatment reduced the crystallinity of UO and TiO
and TiO . The mechanochemically treated powder mixture was heated at 973-1573K for 6 h under Ar atmosphere and analyzed by X-ray diffraction analysis, scanning electron microscopy-energy-dispersive X-ray spectroscopy, and X-ray absorption fine structure analysis. UTi
. The mechanochemically treated powder mixture was heated at 973-1573K for 6 h under Ar atmosphere and analyzed by X-ray diffraction analysis, scanning electron microscopy-energy-dispersive X-ray spectroscopy, and X-ray absorption fine structure analysis. UTi O
O did not form below 1373K without MC treatment and only the starting materials were observed. At 1473 and 1573K, a small amount of UTi
did not form below 1373K without MC treatment and only the starting materials were observed. At 1473 and 1573K, a small amount of UTi O
O and equal amounts of UTi
and equal amounts of UTi O
O and UO
and UO were formed, respectively. The mechanochemically treated sample produced nearly pure UTi
were formed, respectively. The mechanochemically treated sample produced nearly pure UTi O
O containing small amounts of UO
containing small amounts of UO impurities when heated above 1173K for 6 h. UTi
impurities when heated above 1173K for 6 h. UTi O
O was highly crystalline and uniform regardless of the synthesis temperature.
was highly crystalline and uniform regardless of the synthesis temperature.
Ikeda, Yusuke*; Matsumura, Daiju; Tsuji, Takuya; Namai, Asuka*; Imoto, Kenta*; Tokoro, Hiroko*; Nakabayashi, Koji*; Okoshi, Shinichi*
Inorganica Chimica Acta, 550, p.121434_1 - 121434_8, 2023/03
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear) high energy resolution XAFS
high energy resolution XAFSYamamoto, Naoki*; Matsumura, Daiju; Hagihara, Yuto*; Tanaka, Kei*; Hasegawa, Yuta*; Ishii, Kenji*; Tanaka, Hirohisa*
Journal of Power Sources, 557, p.232508_1 - 232508_10, 2023/02
Times Cited Count:2 Percentile:26.88(Chemistry, Physical)Tsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru; Yaita, Tsuyoshi
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(1), p.15 - 18, 2023/02
no abstracts in English
Wakamatsu, Katsuhiro*; Sekihara, Akihori*; Yamaguchi, Yoshihiko*; Matsushima, Ryo*; Matsumura, Daiju; Kuila, T.*; Yoshikawa, Hirofumi*
Batteries & Supercaps (Internet), 6(1), p.e202200385_1 - e202200385_8, 2023/01
Times Cited Count:2 Percentile:26.88(Electrochemistry)Zheng, X.*; Kato, Masaru*; Uemura, Yohei*; Matsumura, Daiju; Yagi, Ichizo*; Takahashi, Kiyonori*; Noro, Shinichiro*; Nakamura, Takayoshi*
Inorganic Chemistry, 62(3), p.1257 - 1263, 2023/01
Times Cited Count:1 Percentile:52.7(Chemistry, Inorganic & Nuclear)Nagai, Takayuki; Tone, Masaya; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2022 (Internet), 3 Pages, 2023/00
no abstracts in English
 O-B
O-B O
O -SiO
-SiO glass plate with bearing NiO for heat center and structural probe
glass plate with bearing NiO for heat center and structural probeTomita, Kana*; Kishi, Tetsuo*; Matsumura, Daiju; Yano, Tetsuji*
Journal of Non-Crystalline Solids, 597, p.121891_1 - 121891_10, 2022/12
Times Cited Count:1 Percentile:13.38(Materials Science, Ceramics)Okamoto, Yoshihiro; Shiwaku, Hideaki; Shimamura, Keisuke*; Kobayashi, Hidekazu; Nagai, Takayuki; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
Journal of Nuclear Materials, 570, p.153962_1 - 153962_13, 2022/11
Simulated nuclear waste glass samples containing phosphorus, which increase the solubility of molybdenum, were prepared and analyzed using synchrotron X-ray Absorption Fine Structure (XAFS) analysis for some constituent elements and Raman spectroscopic analysis of their complex structure. Changes in local structure and chemical state due to different phosphorus additions and waste loading rates were systematically studied. Consequently, no crystalline phase due to the molybdate compound was observed even at a maximum waste content of 30 wt% (corresponding to 1.87 mol% MoO ). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO
). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO structural unit and may deprive the alkali metal coordinated to the molybdate ion.
structural unit and may deprive the alkali metal coordinated to the molybdate ion.